|
Accueil
>
Services
>
Avantor Services
>
Resources
>
Success Stories
>
Cancer Treatment Center Accelerates Research…
Cancer Research Center Accelerates Research in the Face of Safety and Compliance NeedsChallengeFor one of the world’s most renowned cancer treatment and research centers, complying with changing hazardous materials regulations was becoming increasingly difficult. Updating spreadsheet reports annually was insufficient, while maintaining records in real time would impose too great a burden on scientists, taking away time from furthering their research instead. Time was a crucial resource for this institute, which needed to rapidly translate knowledge gained in the laboratory into clinical care, thereby converting discoveries into applications that could save lives. This challenge was just the tip of the iceberg for questions now facing the treatment center, such as:
There were a lot of questions, but not many answers. How could this research center meet the ever-changing regulation requirements and manage improvements to ancillary processes? SolutionAn Avantor Services Business Process Consultant worked with the center’s research administration, materials management, and Environmental, Health, and Safety (EH&S) teams to understand current processes, requirements, and future goals. After analyzing the gathered information from these sources, the consultant designed a best-fit solution that could be integrated into the center’s existing workflow or otherwise outsourced.
ResultA highly effective program was outsourced through a competitive bid process. Following implementation, the program:
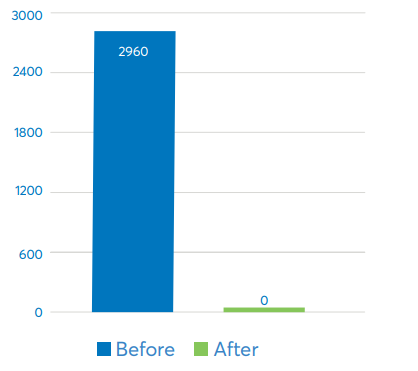
Annual Hours Spent Managing Hazardous Materials DataAvantor Services implemented a system that reduced time spent managing hazardous materials data from 2,960 hours per year to zero hours per year.
ChallengeA prominent cancer treatment and research center needed to adjust to new regulatory requirements for hazardous materials without burdening its vital research community. SolutionThe center engaged Avantor Services to design, implement, and resource a centralized, technology-driven reporting system. ResultThe center has accelerated delivery of accurate reporting data from several days to hours, eliminated the burden of hazardous materials reporting for laboratories, and provided instant access to hazardous materials information for sharing with regulatory agencies. Need Help?Are your scientific resources being wasted on non-research activities? Avantor Services has the skills, knowledge, and experience to support productivity improvement at your organization. |
|
Traitement de votre demande ... |